Dissolving surfaces and degrade –Ending a 70-year debate over the function of enzymes degrading cellulose
Research Group
Taku Uchiyama (Postdoctoral Fellow, Department of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo (at the time of research))
Takayuki Uchihashi (Professor, Research Center for Glycobiology Core Research (iGCORE), Graduate School of Science, Nagoya University, Tokai National University Organization)
Visiting Professor, National Institutes of Natural Sciences, National Institutes of Natural Sciences, Center for the Exploration of Biogenesis)
Takuya Ishida (Postdoctoral Fellow, Department of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo (at the time of research))
Akihiko Nakamura (Associate Professor, Institute for Molecular Science, Cross-Appointment in Life and Coordination Molecular Sciences/Tenure-track Associate Professor, Department of Applied Life Sciences, Faculty of Agriculture, Shizuoka University)
Josh V. Vermaas (Assistant Professor, Department of Biochemistry and Molecular Biology, Michigan State University)
Michael F. Crawly (Center Director, Office of Renewable Energy Research, U.S. Department of Energy)
Masahiro Samejima, Professor Emeritus, Department of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo)
Gregg T. Beckham, Senior Research Fellow, National Renewable Energy Laboratory, U.S. Department of Energy
Kiyohiko Igarashi, Professor, Department of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo/Visiting Professor, VTT Finnish Technical Research Center
key points of the announcement
- We investigated how Lytic Polysaccharide MonoOxygenase (LPMO), an enzyme that oxidatively degrades cellulose, enhances the reactivity of other enzymes.
- It was found that a chain reaction of amorphization occurs around the area oxidized by LPMO on the surface of crystalline cellulose, increasing its degradability by other enzymes.
- To achieve a sustainable and carbon-neutral society, cellulose, the most abundant biomass on earth, must be used for various purposes. The clarification of the details of the cellulose degradation mechanism by enzymes, which is the key to this process, will make it possible to efficiently produce biochemicals, biofuels, bioplastics, and other products from cellulosic biomasses.
Publication Summary
Biochemical experiments have revealed that the degradation of crystalline cellulose by cellulases (Cel6A and Cel7D) produced by wood-rotting fungi is enhanced by the addition of AA9D, a type of Lytic Polysaccharide MonoOxygenase (LPMO). The mechanism was investigated by observation of the enzyme molecules using high-speed atomic force microscopy (Note1) and molecular dynamics simulations (Note2), and it was found that when AA9D oxidatively nicked, not only the reacting cellulose molecules but also the surrounding cellulose molecules were chain wise hydrated and amorphized, enhancing their degradability by other enzymes. This finding is expected to significantly increase the efficiency of producing biochemicals, biofuels, and bioplastics from cellulose, the most abundant biomass on earth, and contribute to achieving a "circular bioeconomy" that is recycling-oriented and biosphere-friendly.
Contents of presentation
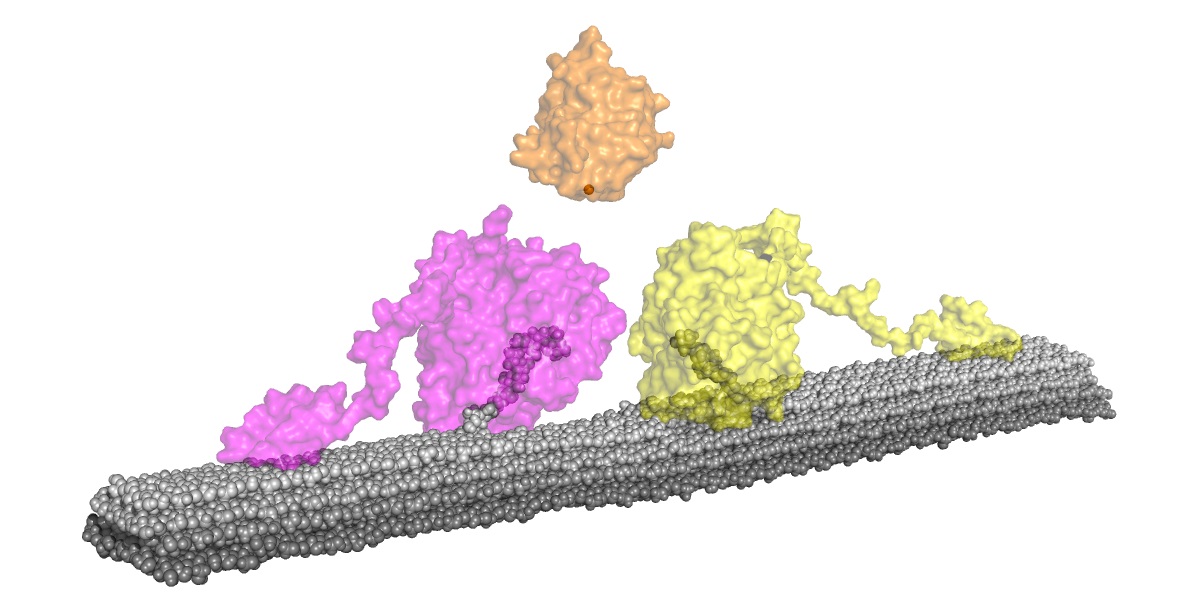
Fig.1 Cellobiohydrolase (Ce6A, yellow Cel7D, peach) degrading the surface of crystalline cellulose oxidized by LPMO (AA9D, orange) from P. chrysosporium.(Enlarged image↗)
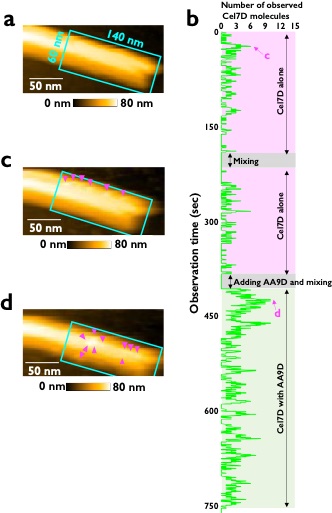
Fig.2 Cellulase molecules observed by high-speed atomic force microscopy.
a: crystalline cellulose before adding enzyme, b: time variation of enzyme molecules, c: view of cellulose surface when only Cel7D is reacting, d: Cel7D and AA9D reacting together. The bright particles are cellulase molecules, and the increase in the number of cellulase molecules observed when AA9D is present can be seen.(movie↗)
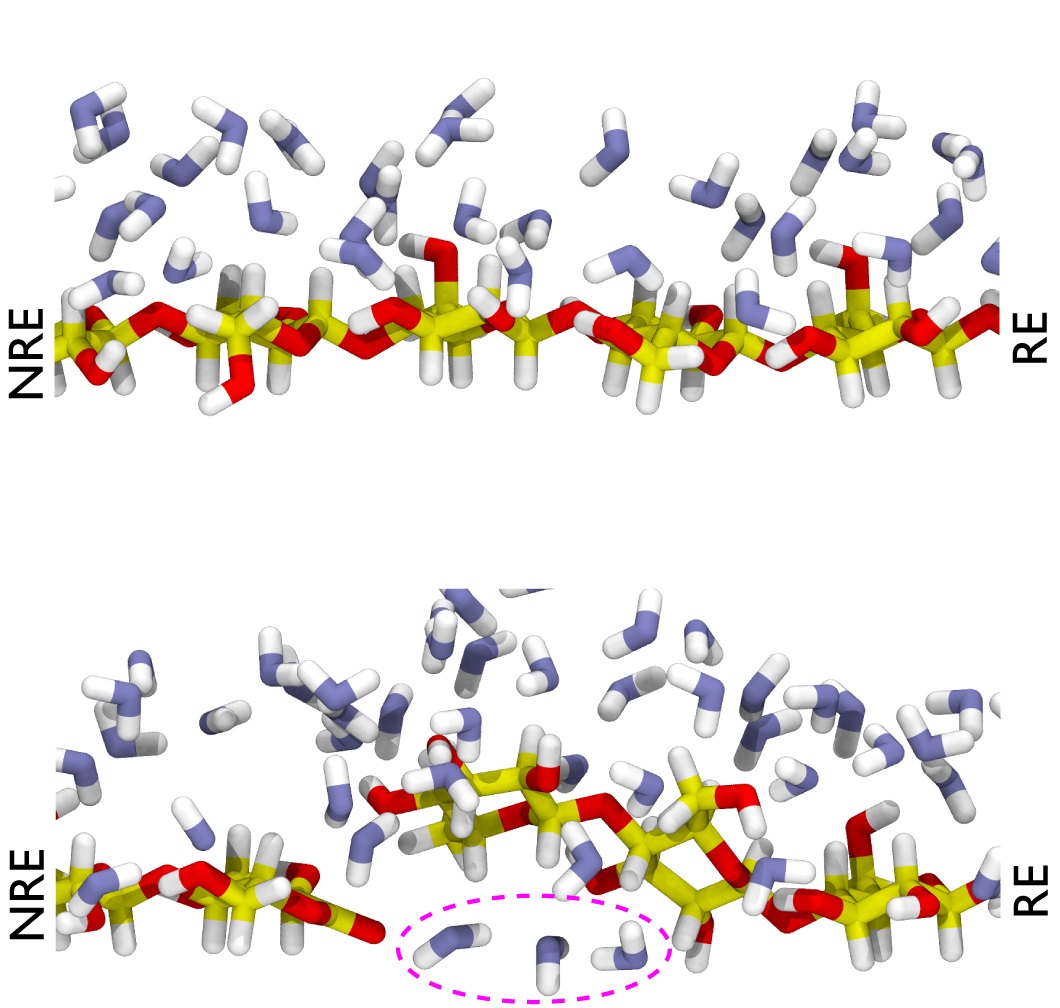
Fig.3 Molecular dynamics of water molecules around the terminal lactone cleaved by AA9D.
The upper row: the result of a molecular dynamics simulation of water molecules around the site where the terminal end of AA9D is cleaved into lactone.
Upper: Surface of crystalline cellulose (control).
Bottom: Crystal surface after cleavage by AA9D. Dotted lines indicate water molecules that flowed under the cellulose molecules.
RE: reducing end, NRE: non-reducing end.
(Enlarged image↗)
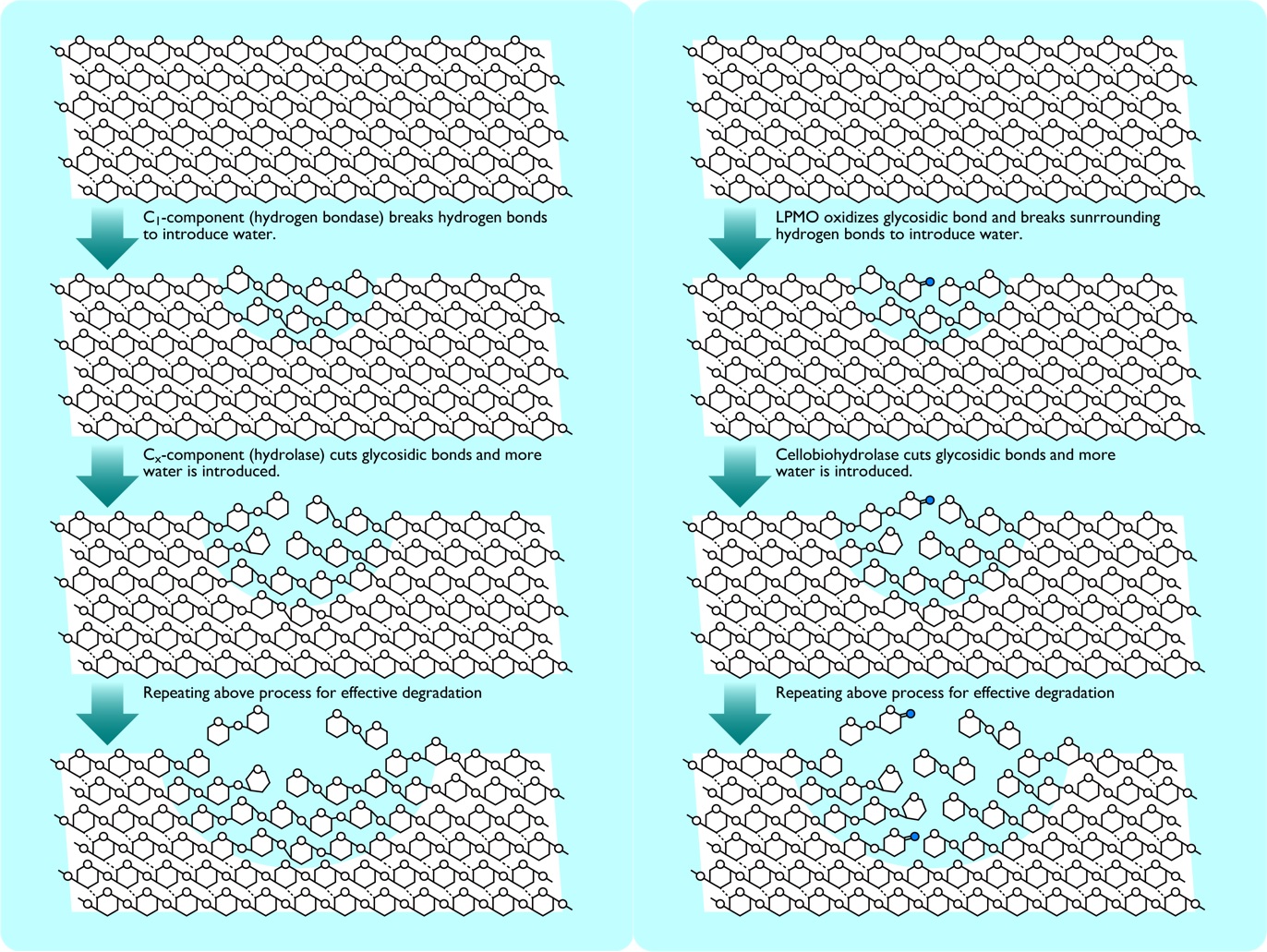
Fig.4 Degradation of cellulose molecules in C1-Cx theory (left) and oxidative boost by LPMO (right).
The process of amorphization by water flow around the area oxidized by LPMO (blue circle) is almost the same as the C1-Cx theory. (Enlarged image↗)
Cellulose is the main component of plant cell walls and the most abundant biomass on earth. Since cellulose is a molecule consisting of thousands to tens of thousands of glucose molecules, if cellulose can be efficiently decomposed, various compounds such as biofuels and biomass plastics can be made from the resulting glucose. However, cellulose is insoluble in water, making it much more difficult to hydrolyze than starch, which is also made up of multiple glucose molecules. On the other hand, there are organisms in nature, such as mushrooms, molds, and bacteria, that efficiently break down cellulose, and use it as an energy source for their growth.
Although studies on enzymatic degradation of cellulose have been conducted since the late 19th century, the mechanism was first discussed by Reese and coworkers at the U. S. Army Natick Development Center in 1950. In that paper, Reese et al. proposed the "C1-Cx theory," in which cellulose is first hydrated by a component called C1 without breaking the glucosidic bonds, and then the hydrated cellulose is hydrolyzed by an enzyme called the Cx component (Ref. 1). However, the enzyme corresponding to the C1 component was not discovered until 20 years later, during which time it became known that cellulose in plant cell walls has both crystalline and amorphous structures (disordered and easily hydrated), and in 1972, mechanism that endo-type enzymes degraded amorphous cellulose, and from there exo-type cellulase breaks down crystalline cellulose from amorphous cellulose (Reference 2), and the endo-exo theory was widely accepted after that. On the other hand, the late Professor Eriksson and coworkers at the University of Georgia, USA, where Professor Kiyohiko Igarashi had studied as a student, discovered in 1974 that cellulose degradation proceeds faster in oxygen-dissolved solutions than in nitrogen-dissolved solutions by reacting cellulase extracted from mushrooms and molds, and proposed "oxidative boost," in which oxidative reactions accelerate cellulose degradation (Reference 3). Subsequently, an enzyme called cellobiose dehydrogenase (CDH) was studied as the causative enzyme, but its mechanism has remained unknown.
In 2010, a Norwegian research team discovered LPMO as the enzyme responsible for the "oxidative boost" (Ref. 4). It was thought to accelerate the oxidative cleavage of cellulose, from which other enzymes react. Later, it was found that CDH can reduce the copper atom in LPMO, and a mechanism involving these redox enzymes in the oxidative boost was proposed.
Prof. Igarashi's team has long studied the enzymatic degradation of crystalline cellulose, and has found that AA9D, an LPMO, produced by the white-rot fungus Phanerochaete chrysosporium was found to have a significant effect on the degradation of crystalline cellulose by the enzymes Cel6A and Cel7D from the same fungus. In the process, they found that AA9D enhances the activity of the other enzymes while producing little of its own product, oxidized oligosaccharides. Then, in collaboration with Prof. Takayuki Uchihashi's research team at Nagoya University, they observed Cel7D molecules working on the cellulose surface by high-speed atomic force microscopy and found that when AA9D was working together, the number of enzyme molecules moving on the cellulose surface increased. Furthermore, in collaboration with Dr. Gregg T. Beckham and his research team at the U.S. Department of Energy's National Renewable Energy Laboratory, they performed molecular dynamics simulations of how the cellulose surface changes when it is oxidized by AA9D, and found that not only cellulose molecules oxidized by AA9D, but also hydrogen bonds with surrounding cellulose molecules are broken, and water molecules flow into them and crystalline cellulose is partly amorphized.
Considering all the above results together, once the LPMO (AA9D in this study) oxidatively cleaves the cellulose, the surrounding hydrogen bond network is disrupted and the unoxidized cellulose is also hydrated and amorphized, which in turn allows other cellulolytic enzymes (Ce6A and Cel7D in this study) to easily degrade the amorphized cellulose molecules, which is thought to cause an "oxidative boost". The mechanism by which the amorphized cellulose is cut from the middle of the molecule is the same as that proposed in the "endo-exo theory," and the amorphization chain of cellulose that begins with "hydration" is the same mechanism as the "C1-Cx theory" proposed by Reese and coworkers in 1950. In other words, these three theories, the C1-Cx theory, the endo-exo theory, and the oxidative boost, were simply looking at the same phenomenon from different perspectives, and this was the decisive factor that put an end to 70 years of debate.
The results of this research can be directly applied to the efficient enzymatic degradation of cellulose, the main component of wood and grass, and can be used to produce biochemicals and bioplastics from unused biomass such as rice straw and sugarcane bagasse (squeezed lees of sugarcane) and cellulose-based biomass such as wastepaper and construction waste. The research results were published in Science Advances, an open-access journal published by the American Association for the Advancement of Science (AAAS).
This research was supported by Grant-in-Aid for Scientific Research on Innovative Areas (18H05484) "Creation of a foundation for sustainable structural systems based on mechano-optimal strategies in plants" (PI: Kiyohiko Igarashi, PD: Prof. Taku Demura, Nara Institute of Science and Technology) and Fundamental Research (B) "Understanding wood rot phenomenon and construction of biomass conversion system using genome editing technology in mushrooms" (19H03013, PI: Kiyohiko Igarashi), "High-speed AFM analysis of structural and mechanical property responses of single proteins with mechanical stimulation" (21H01772, PI: Takayuki Uchihashi), and "Crystallographic analysis of cellulases from actinomycetes" (15K07383, PI: Taku Uchiyama), funded by the Ministry of the Environment and the Environmental Restoration and Conservation Agency (ERCA) (JPMEERF21S11902PI: Kiyohiko Igarashi, PL: Prof. Toshiaki Yoshioka, Tohoku University). The research was supported by the Finland Distinguished Professorship (FiDiPro) program "Advanced approaches for enzymatic biomass utilization and modification (BioAd)" (Invited Professor: Kiyohiko Igarashi) from Business Finland.
Publication Summary
- Journal
- : Science Advances
- Title of pape
- : Lytic polysaccharide monooxygenase increases cellobiohydrolases activity by promoting decrystallization of cellulose surface
- Authors
- : Taku Uchiyama, Takayuki Uchihashi, Takuya Ishida, Akihiko Nakamura, Josh V. Vermaas, Michael F. Crowley, Masahiro Samejima, Gregg T. Beckham, and *Kiyohiko Igarashi (*corresponding author)
- DOI
- : 10.1126/sciadv.ade5155
- : https://www.science.org/doi/10.1126/sciadv.ade5155
For further information, please contact
Department of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo
Professor Kiyohiko Igarashi
Tel:03-5841-5255
E-mail:aquarius<@>g.ecc.u-tokyo.ac.jp Please change
References
- Reese E. T., Sui R. G. H. & Levinson H. S., The biological degradation of soluble cellulose derivatives and its relationship to the mechanism, J. Bacteriol. 59:485-497 (1950)
- Wood T. M. & McCrae S. I. The purification and properties of the C1 component of Trichoderma koningii cellulase, Biochem. J. 128:1183-1192 (1972)
- Eriksson K.-E., Pettersson B., & Westermark U., Oxidation: An important enzyme reaction in fungal degradation of cellulose, FEBS Lett. 49:282-285. (1974)
- Vaaje-Kolstad G., Westereng B., Horn S. J., Liu Z., Zhai H., Sørlie M., Eijsink V. G., An oxidative enzyme boosting the enzymatic conversion, Science 330:219-222 (2010)
Glossary
- Note1 High-speed atomic force microscopy.
The microscope obtains information (images) on the shape of the observed object by scanning a probe along the surface of the object to be observed. However, conventional atomic force microscopes require several minutes to acquire one image. High-speed atomic force microscopy has succeeded in speeding up the scanning time by making various improvements, and can take images in real time. In this study, it is used to observe how cellulase molecules behave on the cellulose surface. - Note2 Molecular dynamics simulation
A method of solving Newton's equations of motion in classical mechanics for each atom constituting a substance and tracking changes in atomic position and energy over time by computer. In this study, we used a molecular dynamics simulation of a material that cannot be observed by high-speed atomic force microscopy.

